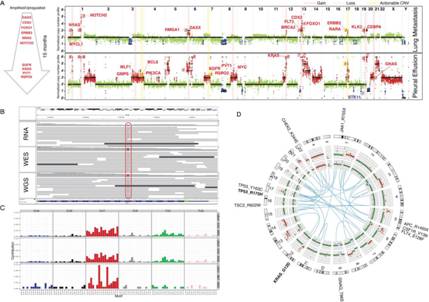
图为胰腺导管癌病人外泌体(exosome)样本的测序分析
癌症的高分辨率综合分析对精准医学至关重要。基于外泌体(exosome)的液体活检可获得高质量的核酸分子特征,这可能对内脏癌症的诊断特别有用,而这些难以通过常规活检来诊断。
MD安德森癌症中心的研究人员从从三个胰胆管癌患者(两个胰腺癌、一个壶腹)的体液分离出外泌体(exosome)。他们用Illumina HiSeq 2500测序仪对exoDNA、exoRNA进行全基因组、外显子组和转录组测序分析。在exoDNA测序数据中检测突变信号和识别潜在可行的突变,以及在exoRNA测序数据中分析表达点突变和基因融合。
全外显子组测序达到95%-99%目标区域的覆盖,平均深度133-490 x。全基因组拷贝数情况,和肿瘤相关片段的高估值(56%-82%),表明外泌体(exosome)中含有肿瘤细胞来源的DNA片段。包括NOTCH1和BRCA2在内的多个可操作的突变在病人exoDNA样本中发现。此外,外泌体(exosome)的RNA测序鉴别了融合基因表达的存在,这为肿瘤新抗原的阐明提供了新的方式。
外泌体(exosome)的核酸成分的拷贝数概况,点突变,插入,缺失,基因融合和突变特征等可作为检测各种各样的肿瘤来源的生物标志物。基于外泌体(exosome)的液体活检可作为临床工具用于癌症诊断,治疗性分层,和治疗监测,这样就不需要直接肿瘤取样,这将大大造福人类健康。
版权归外泌体资讯网所有,欢迎转载,但请注明出处和原文链接!

原文来源:San Lucas, F. A., et al. (2015). "Minimally invasive genomic and transcriptomic profiling of visceral cancers by next-generation sequencing of circulating exosomes." Ann Oncol. IF=7
BACKGROUND: The ability to perform comprehensive profiling of cancers at high-resolution is essential for precision medicine. Liquid biopsies using shed exosomes provide high-quality nucleic acids to obtain molecular characterization, which may be especially useful for visceral cancers that are not amenable to routine biopsies. PATIENTS AND METHODS: We isolated shed exosomes in biofluids from three patients with pancreaticobiliary cancers (two pancreatic, one ampullary). We performed comprehensive profiling of exoDNA and exoRNA by whole genome, exome and transcriptome sequencing using the Illumina HiSeq 2500 sequencer. We assessed the feasibility of calling copy number events, detecting mutational signatures and identifying potentially actionable mutations in exoDNA sequencing data, as well as expressed point mutations and gene fusions in exoRNA sequencing data. RESULTS: Whole exome sequencing resulted in 95 to 99% of the target regions covered at a mean depth of 133 to 490x. Genome-wide copy number profiles, and high estimates of tumor fractions (ranging from 56 to 82%), suggest robust representation of the tumor DNA within the shed exosomal compartment. Multiple actionable mutations, including alterations in NOTCH1 and BRCA2, were found in patient exoDNA samples. Further, RNA sequencing of shed exosomes identified the presence of expressed fusion genes, representing an avenue for elucidation of tumor neoantigens. CONCLUSIONS: We have demonstrated high-resolution profiling of the genomic and transcriptomic landscapes of visceral cancers. A wide range of cancer-derived biomarkers could be detected within the nucleic acid cargo of shed exosomes, including copy number profiles, point mutations, insertions, deletions, gene fusions and mutational signatures. Liquid biopsies using shed exosomes has the potential to be used as a clinical tool for cancer diagnosis, therapeutic stratification, and treatment monitoring, precluding the need for direct tumor sampling.
外泌体资讯网 肿瘤外泌体的下一代测序分析




