近日,韩国研究人员在Journal of Visualized Experiments杂志上发布了一份外泌体电镜的protocol。小编将视频分享给小伙伴们。
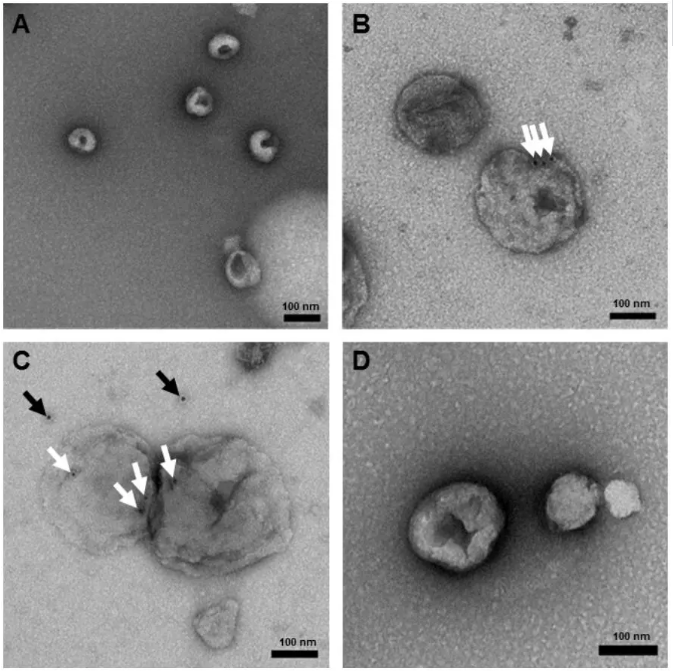
外泌体负染效果图
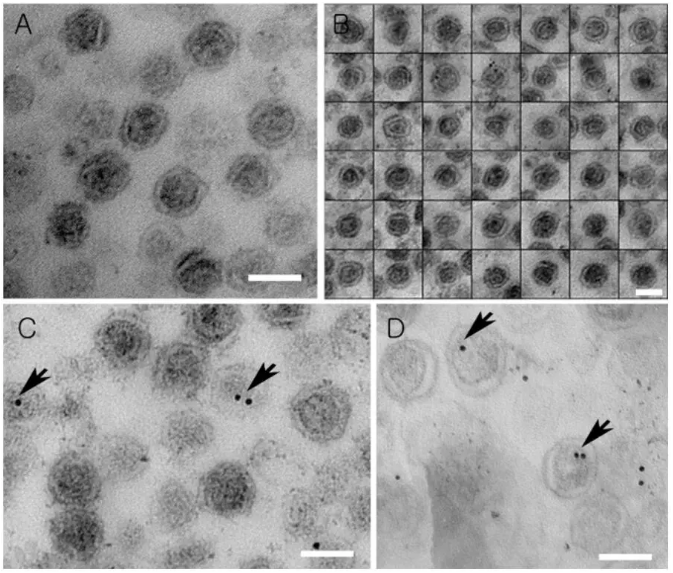 外泌体包埋切片TEM拍摄效果图
外泌体包埋切片TEM拍摄效果图
protocol就不给大家翻译啦,直接贴给大家:
1. Block Preparation, Sectioning, Staining and Imaging of the Exosome
1. Pellet the exosomes from culture supernatant of HCT116 cells by centrifugation at 100,000 x g for 1.5 h23. Remove the culture supernatant
and carefully fix the purified exosome pellet with 1 mL of 2.5% glutaraldehyde in 0.1 M sodium cacodylate solution (pH 7.0) for 1 h at 4 °C.
For 0.1 M sodium cacodylate, dissolve 4.28 g cacodylic acid in 160 mL of distilled water (DW). Adjust pH to 7.4 with 0.1 M HCl then make up
to 200 mL with distilled water.
2. Remove the fixative and rinse the pellets with 1 mL of 0.1 M sodium cacodylate buffer at room temperature. Repeat three times with each change lasting 10 min.
3. Post-fix the samples with 1 mL of 2% Osmium tetroxide for 1 h at 4 °C.
4. Remove the fixative and rinse three times with 0.1 M sodium cacodylate buffer every 10 min.
5. Incubate for 10 min with a graded acetone series (50%, 60%, 70%, 80%, 90%, 95%, 100%, respectively) on the shaker.
6. Remove the acetone and incubate the solution of 3:1 acetone:low viscosity embedding mixture for 30 min. The exosome pellet is in the tube.
7. Remove the medium and add 1:1 acetone:low viscosity embedding mixture medium, then incubate for 30 min.
8. Remove the medium and add 1:3 acetone:low viscosity embedding mixture medium, then incubate for 30 min.
9. Remove the medium and add 100% low viscosity embedding mixture and incubate overnight at room temperature.
10. Embed the sample in pure low viscosity embedding mixture using the embedding mold and bake for 24 h at 65 °C.
11. Prepare sections with 60 nm thickness through an ultra-microtome.
12. Double-stain with 2% uranyl acetate for 20 min and lead citrate for 10 min.
For Reynolds lead solution, add 1.33 g of lead nitrate and 1.76 g of sodium citrate to a total of 50 mL distilled water.
13. Observe the grid under transmission electron microscopy at 80 kV.
14. Click "Acquire" and then click "File" and "Save as" in CCD camera system under the electron microscope at 80 kV. Follow automatic settings
for the exposure time.
2. Immuno-staining of Sections and Imaging
1. Cut 60 nm ultrathin sections using an ultra-microtome, and collect on the nickel grid.
2. Incubate grids in 50 µL drops of 0.02 M glycine for 10 min to quench free aldehyde groups.
3. Rinse in 100 μL of DW three times each for 10 min. Incubate at room temperature for 1 h in PBS containing 1% BSA.
4. Incubate grids in 50 - 100 µL drops of anti KRS antibody24 (1:100 in PBS containing 0.1% BSA) for 1 h (If necessary, this step should be carried out at 4 °C overnight.)
5. Wash grids with five separate drops (50 µL) of PBS containing 0.1% BSA for 10 min each.
6. Transfer grids to drops of 2nd antibody for 1 h (anti-rabbit IgG conjugated to 10 nm gold particle (1:100) in PBS containing 0.1% BSA).
7. Wash grids with five separate drops (50 µL) of PBS containing 0.1% BSA each 10 min.
8. Double-stain with 2% uranyl acetate for 20 min under dark conditions and with Reynold's lead citrate for 10 min, respectively.
9. Click "Acquire" and then click "File" and "Save as" in CCD camera system under a TEM at 80 kV. Exposure time followed automatic settings.
3. Negative Staining
1. Glow-discharge the thin formvar/carbon film coated 200 mesh copper EM grids for 1 min by glow discharger.
2. Fix purified exosomes with 1 mL of 2% Paraformaldehyde (PFA) for 5 min.
Caution: Paraformaldehyde fumes are toxic. All work should be done in a ventilated fume hood.
3. Load 5 - 7 µL exosome suspension solution on the grid and incubate for 1 min. If the concentration of exosome is too high, dilute the concentration to 1/2 - 1/5.
4. Immediately stain with the ~20 drops of filtered 1% uranyl acetate (UA) solution on the surface of the EM grid by syringe.
5. Remove the excess UA solution on the grid by contacting the grid edge with filter paper.
6. Quickly rinse the grid with a drop of water. This step will remove the excess staining solution.
7. Place the grid on the table by holding with tweezers, and cover the grid partially with a culture dish to dry for 10 min under room temperature.
8. Store the grid in an EM grid box for future observation by a TEM at 80 kV.
4. Whole Mount for Immunostaining
1. Glow-discharge the thin formvar/carbon film coated 200 mesh copper EM grids for 30 s.
2. Fix purified exosomes with 1 mL of 2% Paraformaldehyde (PFA) for 5 min.
Caution: Paraformaldehyde fumes are toxic. All work should be done in a ventilated fume hood.
3. Load 5 - 7 µL fixed exosome solution on the grid and incubate for 5 min. Rinse with 100 µL of PBS three times each for 10 min.
4. Treat grids with 50 µL of 0.05 M glycine for 10 min to quench free aldehyde groups.
5. Transfer grids to a drop of blocking buffer (PBS containing 1% BSA) for 30 min.
6. Incubate grids with 50 - 100 µL anti PD-L1 antibody (1:100 in PBS containing 0.1% BSA) for 1 h (if necessary, this step should be carried out at 4 °C overnight).
7. Wash grid with five separate drops (50 µL) of PBS containing 0.1% BSA for 10 min each.
8. Transfer grid to a drop of 2nd antibody for 1 h. Anti-mouse IgG conjugated to 9 - 11 nm gold particle diluted at 1:100 in PBS containing 0.1% BSA.
9. Wash grid with five separate drops (50 µL) of PBS containing 0.1% BSA for 10 min each. Wash grid with two separate drops (50 µL) of DW.
Perform negative staining with 2% uranyl acetate as described from 3.4 to 3.8.
10. Store the grid in an EM grid box for future observation by a TEM at 80 kV.
参考文献:
Jung, M. K., Mun, J. Y. Sample Preparation and Imaging of Exosomes by Transmission Electron Microscopy. J. Vis. Exp. (131), e56482, doi:10.3791/56482 (2018).
外泌体资讯网 【视频】外泌体电镜protocol




